CHOOSE AN IRRESISTIBLE PROTECTION: ATREVIA® ONE FOR ONE MONTH OF PROTECTION AND ATREVIA® XR FOR THREE MONTHS
CONVENIENCE
Choose the most convenient treatment for your patients. Atrevia® One for monthly protection or Atrevia® XR for 3 months of sustained protection against ectoparasites.
FAST AND EFFECTIVE
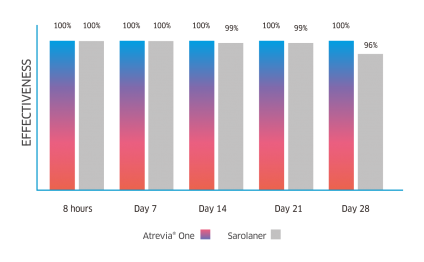
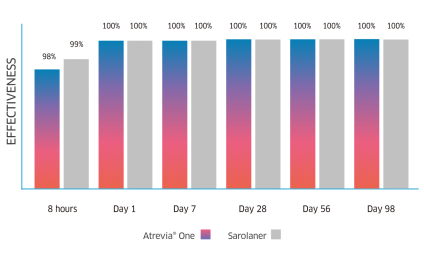
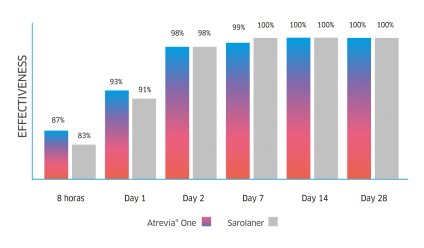
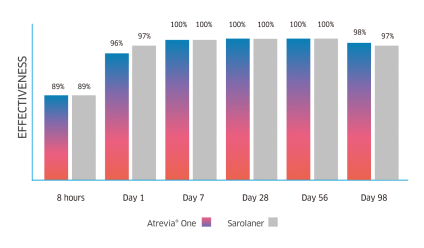
Atrevia® XR acts from the 90th minute against fleas(1), reaching 99% effectiveness at 8 hours after administration(2).
IRRESISTIBLE PROTECTION
Atrevia® has high palatability and acceptance. Acoording to fleas studies, it has a voluntary consumption of 95.65%(3).
BROAD SPECTRUM AND ENVIRONMENTAL CONTROL
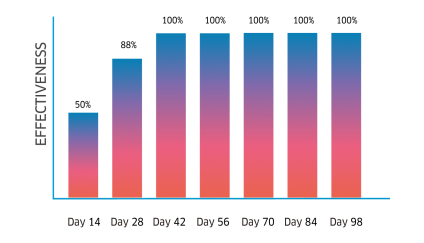
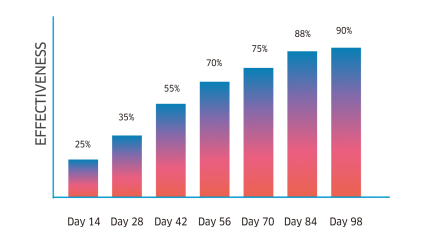
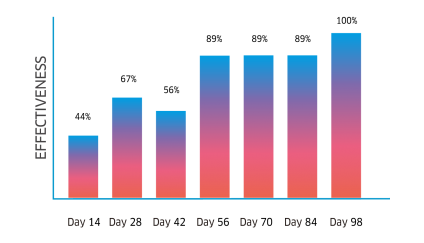
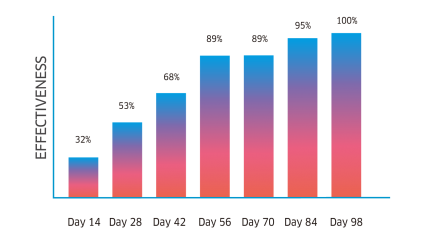
Atrevia® XR is lethal against fleas, ticks, and scabies mites. Its continuous use helps to control inmature stages.
HEALTHIER SKIN
Complete recovery in cases of scabies, and the perfect complement for the treatment against flea allergy dermatitis (FAD).
SAFETY
Safe in lactating females, puppies from the 8th week and/or 2 kg of weight, dogs with MDR1 GEN mutation, and other breeds sensitive to macrocyclic lactones. No serious adverse reactions or neurological reactions have ocurred during the studies.
INNOCUITY
The systemic action of Atrevia® allows protection without exposing families to insecticides as in topical formulations.
WATERRESISTANT
Due to its administration route, it can be used the same bath day. Its efficacy is not diminished by the contact of the pet with water.